Abstract
Introduction:Plasmablastic lymphoma (PBL) is a rare and aggressive lymphoma most commonly seen in the setting of chronic immunosuppression, such as HIV infection and organ transplantation, or in patients with pre-existing lymphoproliferative or autoimmune disorders. PBL commonly presents at extranodal sites and carries a poor prognosis with a global overall survival of 9-15 months after initial diagnosis. Despite poor prognosis for patients with PBL, therapeutic strategies to target this disease are limited, as CHOP-like regimens have failed to produce durable remission, and no standard of care has been established. The cell of origin for PBL is believed to be the plasmablast, as PBL cells possess immunoblastic morphology and contain an immunohistochemical profile positive for plasmablast markers, such as CD38, CD138, and MUM1/IRF4, and negative for B cell markers, such as CD20, CD19, and PAX5. The similarities between PBL cells and multiple myeloma (MM) cells, a plasma cell neoplasm, have led to investigations of the efficacy of MM therapeutics for the treatment of PBL. Daratumumab is a first-in-class monoclonal antibody directed against CD38 that has shown efficacy in treating relapsed/refractory and newly diagnosed MM. Here, we describe the treatment of four patients with advanced-stage PBL in the context of varying degrees of immunosuppression using combination treatment with daratumumab and EPOCH.
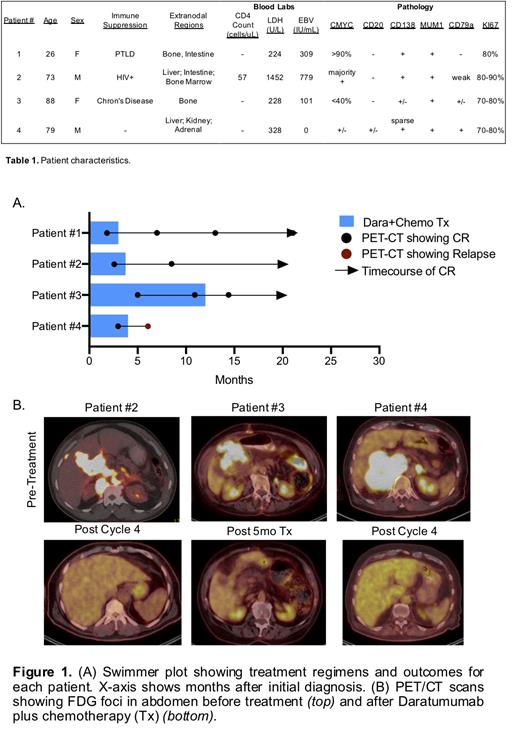
Methods:Four consecutive patients were treated with daratumumab plus chemotherapy. Three of the four patients were treated in the frontline setting and received low-dose EPOCH (vincristine, doxorubicin, etoposide daily for 4 days; cyclophosphamide day 5) with 16mg/kg daratumumab dosed on days 1 and 8 for 6 cycles. Patient #4 showed partial CD20 positivity, prompting Rituximab co-administration (R-EPOCH). Patient #3 was treated in the relapsed setting with 16mg/kg daratumumab in combination with lenalidomide, dexamethasone, and doxorubicin. She was treated for 12 months. Responses were followed by PET/CT imaging.
Results:The four consecutive patients (2 female, 2 male) ranged in age from 26-88 and all had advanced-stage PBL (Table 1). Three of the four patients had some degree of immunosuppression (Patient #1- post-transplantation lymphoproliferative disorder (PTLD), Patient #2- HIV/AIDS, Patient #3- Chron's disease, Patient #4- no history of immunosuppression). All patients had a Ki67 proliferation index over 70% and demonstrated extranodal involvement of disease (bone n=3, intestine n=2, liver n=2; kidney n=1; adrenal n=1).
The three patients that received daratumumab in combination with EPOCH demonstrated a complete response at their first disease assessment by PET/CT scan after cycle 2 (Patient #1) or cycle 4 (Patients #2,4) (Fig1). Patient #3, who demonstrated a mixed response to previous therapy, achieved a complete response 5 months after starting treatment with daratumumab in combination with chemotherapy. As of July 2021, three of the four patients continued to have no evidence of disease for a median of 17 months (range 15-19 months). Patient #4 relapsed in July 2021, 3 months after demonstrating a complete response. Adverse events that required hospitalization were rarely noted following daratumumab treatment but included neutropenic fever (n=2, one event following treatment cycle 4 and the other event 2 months after completion of daratumumab administration), COVID-19 infection (n=1), and a PICC line-associated thrombus (n=1). Minor events were also noted and included self-limiting bradycardia (n=1), neuropathy (n=1), and rigor (n=1).
Conclusions:We describe four patients with varying degrees of immunosuppression and HIV-status with aggressive-stage PBL that achieved complete response following treatment with daratumumab in combination with low-dose EPOCH or other chemotherapy. Three of four patients obtained durable responses. At the time of this writing, three additional HIV +patients have initiated treatment with daratumumab but have not yet reached their first disease assessment. Disease progression for these patients will be monitored and presented as part of this study. Our findings suggest a potential efficacy and warrant further investigation of using daratumumab for the treatment of HIV +and HIV - PBLs.
Amengual: Epizyme, Inc.: Speakers Bureau; Appia Pharmaceuticals: Research Funding; Daiichi Sankyo, Inc: Consultancy; Seagen: Consultancy.
our work uses daratumumab, a first-in-class monoclonal antibody against CD38 for the treatment of plasmablastic lymphoma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal